
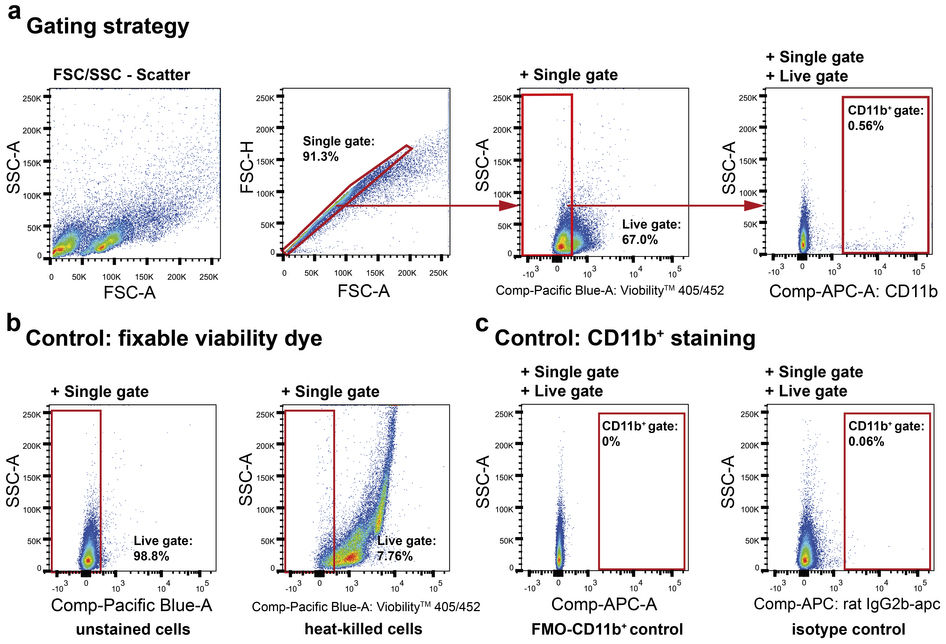
Add 100 μl of cell suspension to each tube.We recommend staining with ice cold reagents/solutions and at 4☌, since low temperature and presence of sodium azide prevent the modulation and internalization of surface antigens which can produce a loss of fluorescence intensity. It is always useful to check the viability of the cells which should be around 95% but not less than 90%. test tubes, eppendorf tubes, and 96-well round-bottomed microtiter plates. However, they can be stained in any container for which you have an appropriate centrifuge e.g. It inhibits metabolic activity.Ĭells are usually stained in polystyrene round-bottom 12 x 75 mm BD Falcon tubes (cat # 352052). if cells are to be collected for functional assays. *Do not add sodium azide to buffers if you are concerned with recovering cell function e.g. Harvest, wash the cells (single cell suspension) and adjust cell number to a concentration of 1-5x106 cells/ml in ice cold FACS Buffer (PBS, 0.5-1% BSA or 5-10% FBS, 0.1% NaN3 sodium azide*).Purity can be calculated by dividing the number of cells in the desired group by the total number of cells sorted. The cell purity can be checked using a microscope or by flow cytometry. After sorting, the cell groups are collected in tubes or plates for further analysis.The SSC channel sorts cells based on their granularity, with more granular cells being sorted into the high SSC channel and less granular cells being sorted into the low SSC channel. The FSC channel sorts cells based on their size, with larger cells being sorted into the high FSC channel and smaller cells being sorted into the low FSC channel. The most common sorting method is called forward scatter (FSC) and side scatter (SSC).

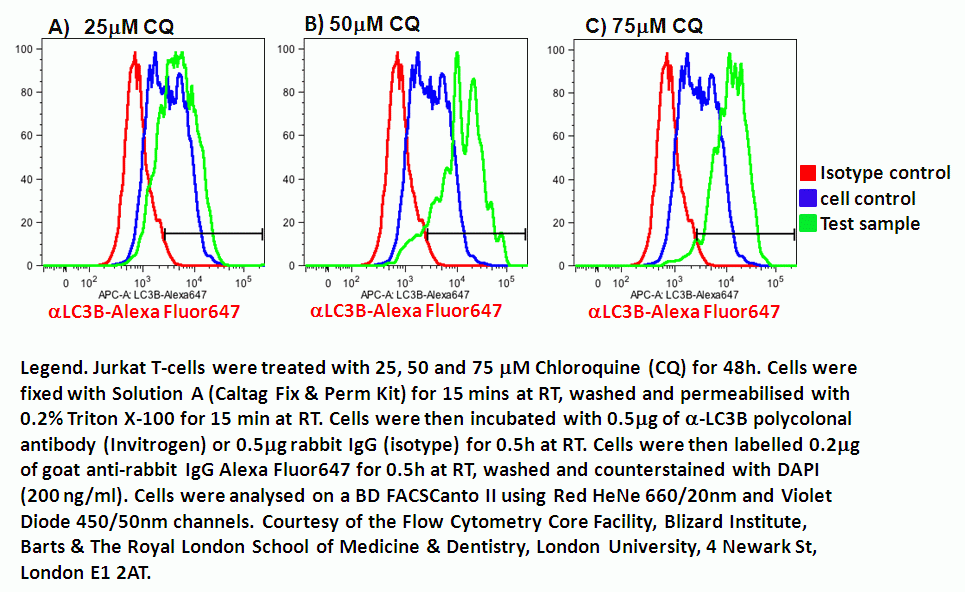
The cells are sorted into groups according to their fluorescence intensity and size.Based on the fluorescence intensity, the flow cytometer sorts the cells into different groups. The flow cytometer uses lasers to excited the fluorescent dyes and detectors to measure the fluorescence intensity of the dyes. The next step is to pass the labeled cells through a flow cytometer.The cells are then resuspended in phosphate buffered saline (PBS) to remove unbound dye. The cell sorting buffer contains salts, proteins, and other molecules that help to keep the cells healthy during the sorting process. After incubation, the cells are washed and diluted using a buffer.The cells are incubated with the fluorescent dyes for 30-60 minutes. FITC is a green fluorescent dye that is excited by blue light and PE is a red fluorescent dye that is excited by green light. Cells are routinely labelled with PE or FITC conjugated antibodies. The fluorescent dyes are usually attached to antibodies that bind to specific cell surface markers. The first step in FACS sorting is to label the cells with fluorescent dyes.Finally, FACS can be used to sort cells into multiple groups, allowing for a more detailed analysis of cell populations.įluorescent activated cell sorting is a complex procedure and requires trained personnel and specialized equipment. Secondly, FACS can be used to purify cells that are difficult to separate using other methods. FACS is much faster than these methods and can often be completed in minutes. This allows for the characterization of cell populations.įACS offers many benefits over traditional cell separation techniques, such as centrifugation and filtration. Cells can be analyzed for their size, shape, fluorescence intensity, and other parameters. Cell analysis is the final benefit of FACS. This allows for the detection and quantification of specific proteins or DNA sequences. Cells can be labeled with fluorescent dyes that bind to specific proteins or DNA sequences. Commonly used surface markers include CD markers such as CD45 (a leukocyte marker), CD14 (a monocyte marker), and HLA-DR (a T-cell marker).Ĭell labelling is another common process performed using FACS. Cell separation is the most common application of FACS, which is carried out based on a variety of parameters, including cell size, shape, and surface markers. The main benefits of FACS are cell separation, cell labeling, and cell analysis. FACS is carried out using a flow cytometer, which is a machine that can measure the fluorescence of cells as they pass through a laser beam. FACS is based on the principle that cells can be labeled with fluorescent dyes and sorted according to their fluorescence intensity. Fluorescence activated cell sorting (FACS) is a technique that can be used to isolate specific cell types from a mixed population.


 0 kommentar(er)
0 kommentar(er)
